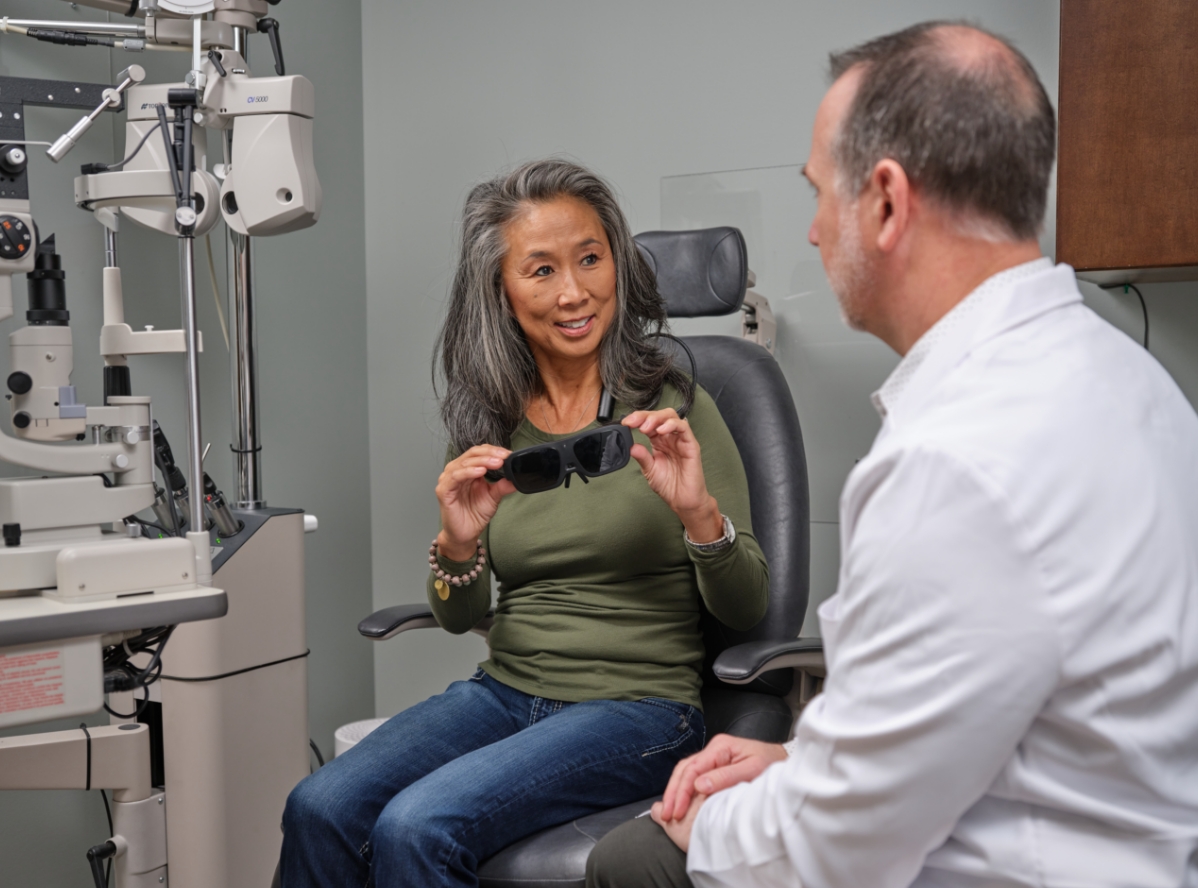
Deliver eSight® To Your Patients via Telehealth
eSight is a clinically validated, FDA-registered low vision device designed to help your patients with central vision loss achieve enhanced visual acuity.
eSight® – A proven low vision device for your patients with central vision loss
eSight enables patients with central vision loss to achieve enhanced visual acuity. It works by stimulating the synaptic nerve activity in the remaining photoreceptor function in the patient’s eyes to provide increased visual information to the brain. In doing so, eSight delivers life-changing results for thousands of people with macular degeneration, diabetic retinopathy, Stargardt disease and more.
eSight provides unprecedented improvement in a wide range of visual performance measures for patients with one of over 20 different eye conditions associated with central vision loss.¹ eSight is a class 1 medical device that is registered with the FDA and EUDAMED, and inspected by Health Canada.


eSight® Is Clinically Validated Through A Peer-Reviewed Multicenter Trial
A multisite, prospective, single-arm study that involved six recruitment centers investigated the short-and medium-term effects of eSight. With a sample size of n=51, this study primarily demonstrated:
- A seven-line gain in distance acuity
- 100% mobility retention
- 12 letter contrast improvement
- Improvement in critical print size and reading acuity
- Significant increase in facial recognition
eSight eyewear significantly improves distance acuity
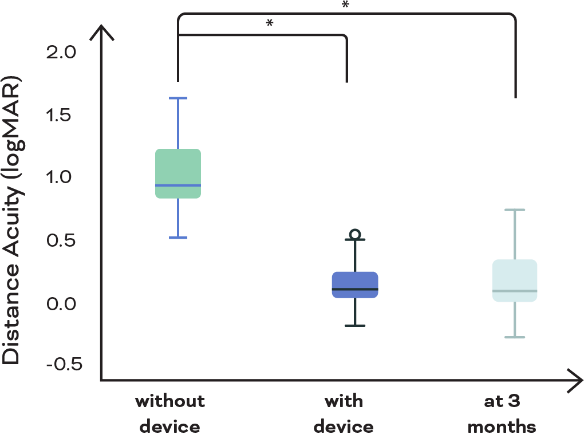
Figure 1. Distance acuity equivalent of print size correctly read on the ETDRS chart at baseline without the device, at fitting with the device, and after 3 months of device use. Mean baseline acuity without device was 20/177 (mean logMAR, 0.95 [SD, 0.25]), which improved to 20/32 (mean, 0.20 [SD, 0.31]) with the device but stayed unchanged after 3 months of device use and training (mean, 0.19 [SD, 0.30]). *Statistically significant differences. ETDRS = Early Treatment Diabetic Retinopathy Study.
eSight eyewear significantly improves reading acuity
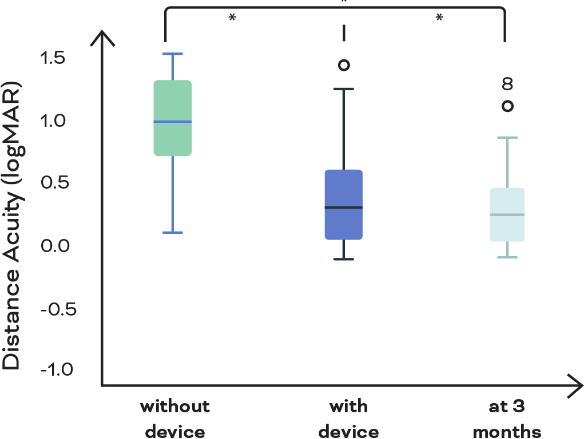
Figure 2. Reading acuity equivalent of print size read on the MNREAD chart at baseline without the device, at fitting with the device, and after 3 months of device use. Mean baseline reading acuity without device was 20/159 (mean logMAR, 0.90 [SD, 0.34]), which improved to 20/43 (mean, 0.33 [SD, 0.39]) with the device but stayed unchanged after 3 months of device use and training (mean, 0.24 [SD, 0.36]). *Statistically significant differences.
Our eQuest Partners







Experience The Ease Of eSight TeleHealth
Refer your patients to eSight Telehealth, and eSight specialists will ensure eligibility through an in-home evaluation of the device via TeleHealth. With the support of the TeleHealth training programs, eSight is efficient, reduces travel time, and offers a seamless, patient-centered experience from referral to usage.
- An appointment with an eSight clinical specialist who will train and guide patients on using the device
- The use of an eSight device for several days for evaluation after the training session
- No cost or financial obligation to the patient with your referral







